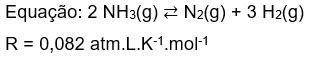Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
4) Considere que em condições de equilíbrio as pressões parciais de NH₃(g), N₂(g) e H₂(g) são respec...
Questions

Chemistry, 11.03.2021 23:20


Mathematics, 11.03.2021 23:20


Physics, 11.03.2021 23:20


Mathematics, 11.03.2021 23:20


Mathematics, 11.03.2021 23:20


Chemistry, 11.03.2021 23:20



Mathematics, 11.03.2021 23:20




Chemistry, 11.03.2021 23:20


Mathematics, 11.03.2021 23:20