
Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solution of salt in contact with a solution containing a common ion. Show that changes in the initial concentrations:
PbCl2(s) in 0.631 M MgCl2 (MgCl2 is strong electrolyte)
Ksp PbCl2 = 1.6 × 10−5

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Assuming that no equilibria other than dissolution are involved, calculate the concentration of all...
Questions




Spanish, 11.12.2021 01:00


Social Studies, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00


English, 11.12.2021 01:00


Advanced Placement (AP), 11.12.2021 01:00

Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00


Mathematics, 11.12.2021 01:00





![[Mg^{2+} ] = 0.631 M \\ [Pb] = 1.0 \times 10^{-5} M \\[Cl^{-} ] = 1.26 M](/tpl/images/1407/4448/37a02.png)
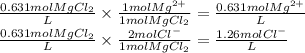
![Ksp = 1.6 \times 10^{-5} = [Pb^{2+} ][Cl^{-} ]^{2} = (x) (1.26+x)^{2}](/tpl/images/1407/4448/5a884.png)
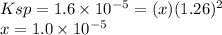
![[Mg^{2+} ] = 0.631 M\\ [Pb] = x = 1.0 \times 10^{-5} M\\[Cl^{-} ] = 1.26+x = 1.26 M](/tpl/images/1407/4448/f1396.png)


