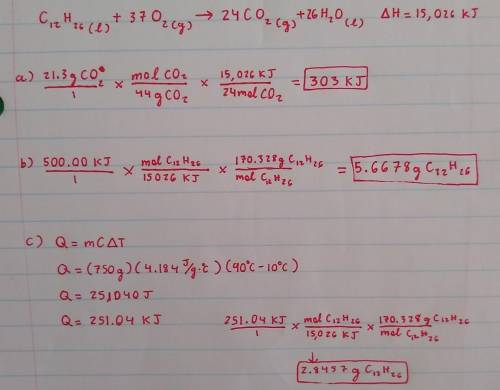Chemistry, 10.08.2021 07:10 yourmumsanoodle
1. Consider the following thermochemical reaction for kerosene:
2 C12H26(l) + 37 O2(g) 24 CO2(g) + 26 H2O(l) + 15,026 kJ
(a) When 21.3 g of CO2 are made, how much heat is released?
(b) If 500.00 kJ of heat are released by the reaction, how grams of C12H26 must have been consumed ?
(c) If this reaction were being used to generate heat, how many grams of C12H26 would have to be reacted to generate
enough heat to raise the temperature of 750g of liquid water from 10oC to 90oC?
2. Consider the reaction: NaNO3(s) + H2SO4(l) → NaHSO4(s) + HNO3(g) ΔH° = 21.2 kJ
How much heat must absorbed by the reaction system to convert 100g of NaNO3 into NaHSO4(s)?
3. What is the enthalpy change when 49.4 mL of 0.430 M sulfuric acid reacts with 23.3 mL of 0.309 M potassium
hydroxide?
3.
H2SO4(aq) + 2KOH(aq) → K2SO4(aq) + 2H2O(l) ΔH° = –111.6 kJ/mol

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
1. Consider the following thermochemical reaction for kerosene:
2 C12H26(l) + 37 O2(g) 24 CO2(g)...
Questions

Chemistry, 18.07.2019 04:50

History, 18.07.2019 04:50


Computers and Technology, 18.07.2019 04:50

Physics, 18.07.2019 04:50

History, 18.07.2019 04:50


Social Studies, 18.07.2019 04:50



Mathematics, 18.07.2019 04:50


Biology, 18.07.2019 04:50

Mathematics, 18.07.2019 04:50

Mathematics, 18.07.2019 04:50

Chemistry, 18.07.2019 04:50

History, 18.07.2019 04:50