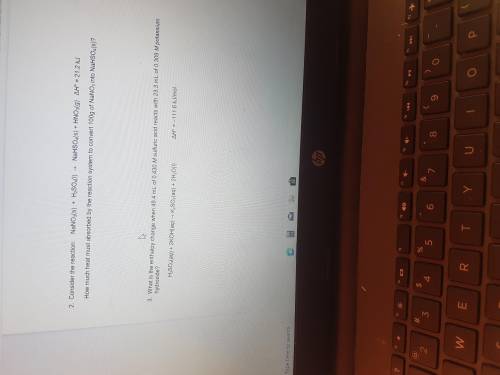
NaNO3 (s)+H2SO4(l)-NaHSO4(s)+HNO3(g)
Delta H= 21.2kj
How much heat must be absorbed by...

Chemistry, 08.08.2021 04:10 wolfiewolffromsketch
NaNO3 (s)+H2SO4(l)-NaHSO4(s)+HNO3(g)
Delta H= 21.2kj
How much heat must be absorbed by the reaction system to convert 100g of NaSO3 into NaHSso4(s)?

Answers: 2


Another question on Chemistry




Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
Questions

Biology, 12.09.2019 18:10

English, 12.09.2019 18:10

Mathematics, 12.09.2019 18:10

Mathematics, 12.09.2019 18:10





Health, 12.09.2019 18:10






Mathematics, 12.09.2019 18:10

Mathematics, 12.09.2019 18:10


Biology, 12.09.2019 18:10

English, 12.09.2019 18:10



