
Chemistry, 07.08.2021 03:00 korireidkdotdot5973
The half-reaction at the cathode in an electrochemical cell is given below.
What other half-reaction would most likely occur at the anode to produce a
spontaneous reaction?
Zn2+(aq) + 2e —> Zn(s)
A. H2(g) —> 2H+(aq) + 2e-
B. Al(s) —> Al3+ (aq) + 3e-
C. K^+ (aq) + e^- —> K (s)
D. Zn (s) —> Zn2^+ (aq) + 2e-
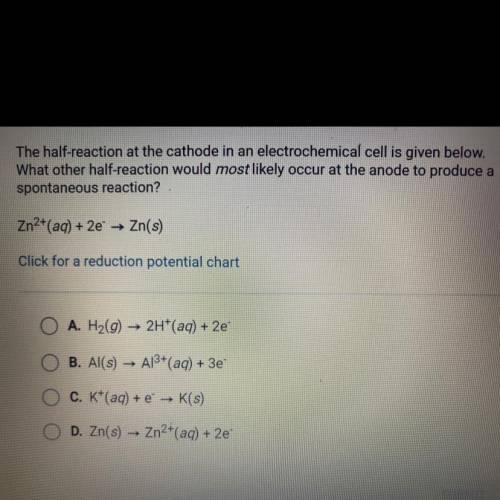

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1

Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1
You know the right answer?
The half-reaction at the cathode in an electrochemical cell is given below.
What other half-reactio...
Questions

Social Studies, 26.06.2019 16:30


Mathematics, 26.06.2019 16:30



Mathematics, 26.06.2019 16:30

Social Studies, 26.06.2019 16:30

Mathematics, 26.06.2019 16:30




Chemistry, 26.06.2019 16:30

Geography, 26.06.2019 16:30


Mathematics, 26.06.2019 16:30

Mathematics, 26.06.2019 16:30


Mathematics, 26.06.2019 16:30

Mathematics, 26.06.2019 16:30



