Chemistry, 06.08.2021 21:40 aspenbaxter201634
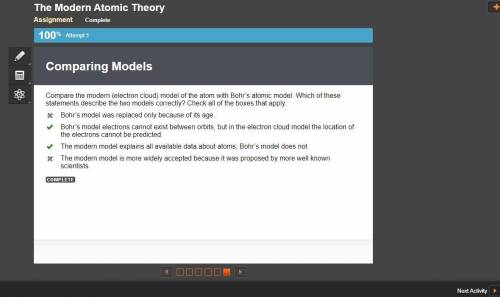
Compare the modern (electron cloud) model of the atom with Bohr’s atomic model. Which of these statements describe the two models correctly? Check all of the boxes that apply.
A. Bohr’s model was replaced only because of its age.
B. Bohr’s model electrons cannot exist between orbits, but in the electron cloud model the location of the electrons cannot be predicted.
C. The modern model explains all available data about atoms; Bohr’s model does not.
D. The modern model is more widely accepted because it was proposed by more well known scientists.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1


Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
Compare the modern (electron cloud) model of the atom with Bohr’s atomic model. Which of these state...
Questions

History, 29.11.2021 18:50


Geography, 29.11.2021 18:50

Mathematics, 29.11.2021 18:50

Mathematics, 29.11.2021 18:50

Mathematics, 29.11.2021 18:50


Physics, 29.11.2021 18:50

Mathematics, 29.11.2021 18:50



Mathematics, 29.11.2021 18:50

Computers and Technology, 29.11.2021 18:50



Mathematics, 29.11.2021 18:50



Chemistry, 29.11.2021 18:50

World Languages, 29.11.2021 18:50