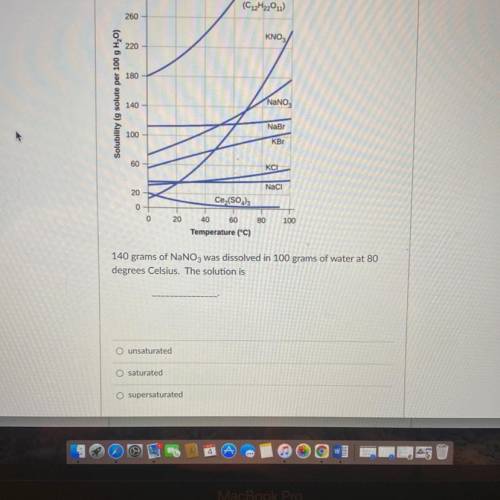
Temperature (°C)
140 grams of NaNO3 was dissolved in 100 grams of water at 80
degrees Celsius...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2


Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Questions


Chemistry, 05.10.2020 01:01

Mathematics, 05.10.2020 01:01

History, 05.10.2020 01:01

Computers and Technology, 05.10.2020 01:01

History, 05.10.2020 01:01


Mathematics, 05.10.2020 01:01



Chemistry, 05.10.2020 01:01

Mathematics, 05.10.2020 01:01

Computers and Technology, 05.10.2020 01:01

Mathematics, 05.10.2020 01:01


Arts, 05.10.2020 01:01

Chemistry, 05.10.2020 01:01

History, 05.10.2020 01:01