Chemistry, 04.08.2021 17:00 wdgyvwyv8840
A stock solution of magnesium chloride has a concentration of 120 mg mL. How many milliliters of the stock solution are required to prepare 1.5 L of 25 mg mL solution

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
A stock solution of magnesium chloride has a concentration of 120 mg mL. How many milliliters of the...
Questions



Social Studies, 25.02.2020 18:00



Health, 25.02.2020 18:00






History, 25.02.2020 18:00




Physics, 25.02.2020 18:00


English, 25.02.2020 18:00


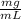 Vi= ?Cf= 1.5 L= 1500 mL (beign 1 L= 1000 mL)Vf= 25
Vi= ?Cf= 1.5 L= 1500 mL (beign 1 L= 1000 mL)Vf= 25