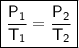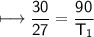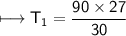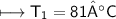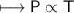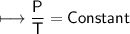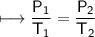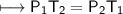Chemistry, 03.08.2021 14:10 Vickyvics4113
The gases in a hair spray can are at a temperature of 27oC and a pressure of 30 lbs/in2. If the
gases in the can reach a pressure of 90 lbs/in2, the can will explode. To what temperature must
the gases be raised in order for the can to explode? Assume constant volume. Show your work.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
The gases in a hair spray can are at a temperature of 27oC and a pressure of 30 lbs/in2. If the
gas...
Questions


Social Studies, 15.02.2021 22:30





World Languages, 15.02.2021 22:30





Health, 15.02.2021 22:30

Arts, 15.02.2021 22:30

Mathematics, 15.02.2021 22:30


Chemistry, 15.02.2021 22:30



Computers and Technology, 15.02.2021 22:30

Mathematics, 15.02.2021 22:30