
Chemistry, 02.08.2021 19:30 salinasroel22
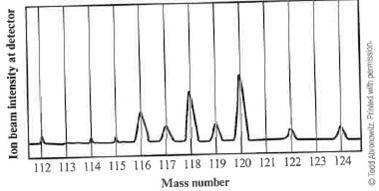
When a sample of an unknown element is vaporized and injected into a mass spectrometer, the results shown below are obtained. Use these data to estimate the average atomic mass of this element.
(A) 117 amu
(B) between 117 and 118 amu
(C) between 118 and 119 amu
(D) between 119 and 120 amu

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
When a sample of an unknown element is vaporized and injected into a mass spectrometer, the results...
Questions




History, 23.09.2019 06:00

Mathematics, 23.09.2019 06:00

Biology, 23.09.2019 06:00

Mathematics, 23.09.2019 06:00


Geography, 23.09.2019 06:00

Physics, 23.09.2019 06:00







Mathematics, 23.09.2019 06:00


Mathematics, 23.09.2019 06:00



