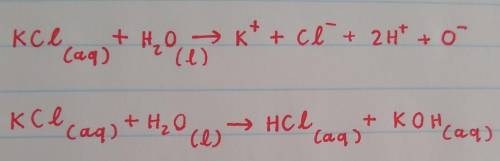When KCl dissolves in water:
the Cl- ions are attracted to the partially negative oxygen atoms of the water molecule.
the K+ ions are attracted to Cl- ions on the KCl crystal.
the K+ ions are attracted to the partially positive hydrogen atoms of the water molecule.
the K+ ions are attracted to the partially negative oxygen atoms of the water molecule.
the Cl- ions are attracted to dissolved K+ ions.
Please explain!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

You know the right answer?
When KCl dissolves in water:
the Cl- ions are attracted to the partially negative oxygen atoms of t...
Questions

Mathematics, 22.09.2021 09:40






Computers and Technology, 22.09.2021 09:40


English, 22.09.2021 09:40

Mathematics, 22.09.2021 09:40

Mathematics, 22.09.2021 09:40




Mathematics, 22.09.2021 09:40

Mathematics, 22.09.2021 09:40

Mathematics, 22.09.2021 09:40


Mathematics, 22.09.2021 09:40