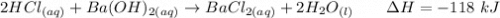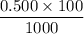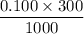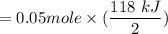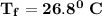Chemistry, 30.07.2021 23:00 maskythegamer
Calculate the heat when 100.0 mL of 0.500 M HCl is mixed with 300.0 mL of 0.100 M Ba(OH)2. Assuming that the temperature of both solutions was initially 25.0C and that the final mixture has a mass of 400.0 g and a specific heat capacity of 4.18 J/C g, calculate the final temperature of the mixture.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Calculate the heat when 100.0 mL of 0.500 M HCl is mixed with 300.0 mL of 0.100 M Ba(OH)2. Assuming...
Questions


History, 11.11.2019 19:31

History, 11.11.2019 19:31

Social Studies, 11.11.2019 19:31

History, 11.11.2019 19:31



History, 11.11.2019 19:31

Chemistry, 11.11.2019 19:31

Geography, 11.11.2019 19:31

English, 11.11.2019 19:31

Biology, 11.11.2019 19:31



Mathematics, 11.11.2019 19:31





Geography, 11.11.2019 19:31