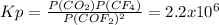Chemistry, 30.07.2021 02:40 blazecarley
Predict whether reactants or products will be favored at equilibrium for the below reaction.
Kp= 2.2 x 10^6 at 298K
2COF2 (g) + ⇌ CO2(g) + CF4(g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Predict whether reactants or products will be favored at equilibrium for the below reaction.
Kp= 2....
Questions


Mathematics, 26.04.2021 22:00


Mathematics, 26.04.2021 22:00




Mathematics, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00




Mathematics, 26.04.2021 22:00


Social Studies, 26.04.2021 22:00


Mathematics, 26.04.2021 22:00

Arts, 26.04.2021 22:00