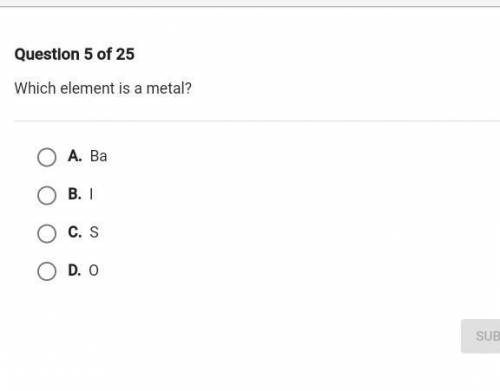
PLZ HELL ME WITH MY WORK
...

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Questions




Mathematics, 18.06.2020 03:57

Social Studies, 18.06.2020 03:57


Chemistry, 18.06.2020 03:57


English, 18.06.2020 03:57

English, 18.06.2020 03:57

Mathematics, 18.06.2020 03:57

Mathematics, 18.06.2020 03:57

Mathematics, 18.06.2020 03:57

English, 18.06.2020 03:57




Medicine, 18.06.2020 03:57

Mathematics, 18.06.2020 03:57