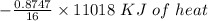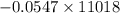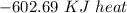Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

You know the right answer?
Using the following equation for the combustion of octane calculate the heat associated with the for...
Questions










Mathematics, 06.12.2019 22:31

Chemistry, 06.12.2019 22:31

Mathematics, 06.12.2019 22:31







History, 06.12.2019 22:31

English, 06.12.2019 22:31

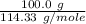
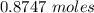
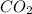 formation associates with -11018 kJ of heat, then
formation associates with -11018 kJ of heat, then