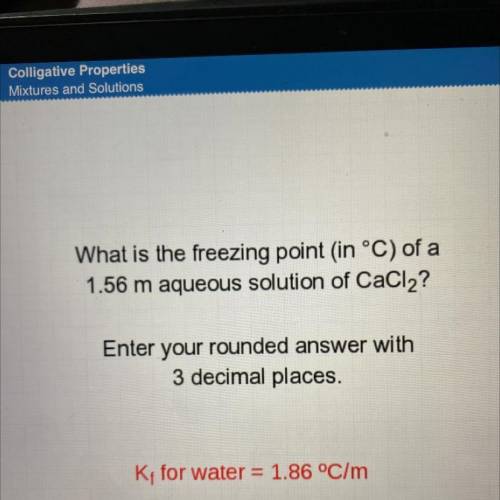
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded a...

Chemistry, 26.07.2021 22:20 joselinegarciaowyrpf
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded answer with
3 decimal places.
K; for water = 1.86 °C/m

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Which of these reactions are redox reactions? check all that apply.cd + hcl → cdcl2 + h2cucl2 + na2s → 2nacl + cuscaco3 → cao + co2 2zns + 3o2 → 2zno + 2so2 ch4 + 2o2 → co2 + 2h2o
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Questions








Engineering, 26.07.2019 19:30










Biology, 26.07.2019 19:30




