
150 mL of 0.25 mol/L magnesium chloride solution and 150 mL of 0.35 mol/L silver nitrate solution are mixed together. After reaction is completed; calculate the concentration of nitrate ions in solution. Assume that the total volume of the solution is 3.0 x 10^2 mL

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
150 mL of 0.25 mol/L magnesium chloride solution and 150 mL of 0.35 mol/L silver nitrate solution ar...
Questions



Mathematics, 25.11.2021 07:30



Computers and Technology, 25.11.2021 07:30


Computers and Technology, 25.11.2021 07:30

Mathematics, 25.11.2021 07:30

Computers and Technology, 25.11.2021 07:30


Mathematics, 25.11.2021 07:30

Mathematics, 25.11.2021 07:30

Social Studies, 25.11.2021 07:30



Mathematics, 25.11.2021 07:30



Mathematics, 25.11.2021 07:30

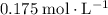 .
.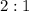 ratio:
ratio: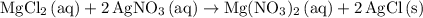 .
.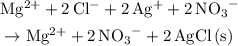 .
. 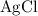 is insoluble in water and barely ionizes. Hence,
is insoluble in water and barely ionizes. Hence, 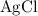 isn't rewritten as ions.
isn't rewritten as ions.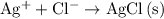 .
.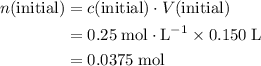 .
.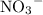 do not take part in any reaction in this mixture, the quantity of this ion would stay the same.
do not take part in any reaction in this mixture, the quantity of this ion would stay the same. 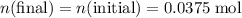 .
.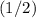 of the concentration in the original solution.
of the concentration in the original solution.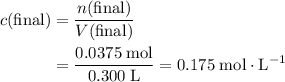 .
.

