
Chemistry, 24.07.2021 21:00 mickimlvn6128
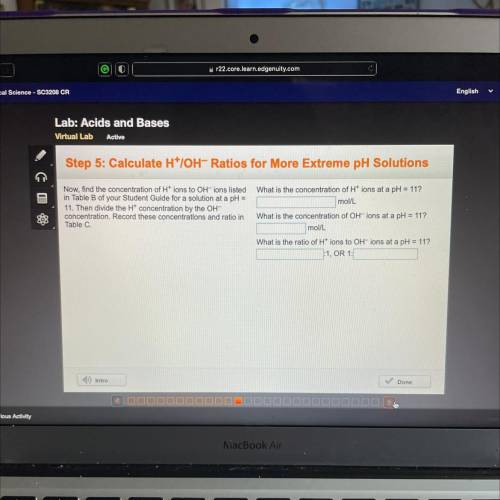
Step 5: Calculate H*IOH-Ratios for More Extreme pH Solutions
What is the concentration of H+ ions at a pH = 11?
mol/L
Now, find the concentration of H+ ions to OH-ions listed
in Table B of your Student Guide for a solution at a pH =
11. Then divide the Ht concentration by the OH-
concentration. Record these concentrations and ratio in
Table C.
What is the concentration of OH-ions at a pH = 11?
mol/L
What is the ratio of H+ ions to OH-ions at a pH = 11?
:1, OR 1:

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 12:30
Growing crops in places where major pests don't live using beneficial insects to eat harmful insects using a rat trap instead of a rodenticide developing drought-resistant tomato plants using beneficial insects or natural oils to repel pests planting a different crop every year to fake out the pests keeping food covered to deter ants and rodents developing bean plants with a resistance to fungus picking caterpillars off tomato plants cultivation practice biological control cultural control genetic resistance natural chemicals
Answers: 3
You know the right answer?
Step 5: Calculate H*IOH-Ratios for More Extreme pH Solutions
What is the concentration of H+ ions a...
Questions

Mathematics, 10.10.2019 15:10


Mathematics, 10.10.2019 15:10

History, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10




Mathematics, 10.10.2019 15:10


Health, 10.10.2019 15:10

Biology, 10.10.2019 15:10

English, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10

Physics, 10.10.2019 15:10

History, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10




