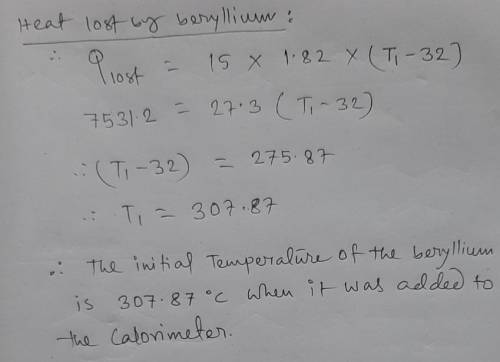Chemistry, 24.07.2021 01:00 edandjill24
The specific heat of beryllium is 1.82 J/g*K, and tou have a mass of 15.0 g. When the beryllium is heated it is added to a calorimeter with a 150.0 g of water (initial temperature of water is 20.0 degrees Celsius) the system reaches equilibrium at 32.0 degrees Celsius. What was the initial temperature of the beryllium when it was added to the colorimeter?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

You know the right answer?
The specific heat of beryllium is 1.82 J/g*K, and tou have a mass of 15.0 g. When the beryllium is h...
Questions

Geography, 21.04.2021 06:00


Mathematics, 21.04.2021 06:00

Mathematics, 21.04.2021 06:00

History, 21.04.2021 06:00


Spanish, 21.04.2021 06:00

History, 21.04.2021 06:00

Physics, 21.04.2021 06:00




Mathematics, 21.04.2021 06:00




Mathematics, 21.04.2021 06:00



History, 21.04.2021 06:00