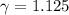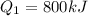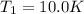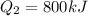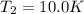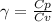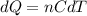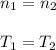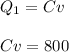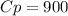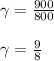A quantity of ideal gas requires 800 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant volume. The same quantity of gas requires 900 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant pressure. What is the adiabatic gas constant of this gas

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
A quantity of ideal gas requires 800 kJ to raise the temperature of the gas by 10.0 K when the gas i...
Questions


Mathematics, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20

Social Studies, 26.01.2021 18:20


Mathematics, 26.01.2021 18:20




Arts, 26.01.2021 18:20

Chemistry, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20

English, 26.01.2021 18:20




Mathematics, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20

English, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20