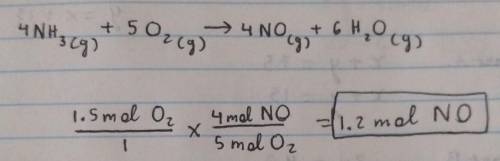Gaseous ammonia chemically reacts with oxygen gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of nitrogen monoxide produced by the reaction of 1.5 moles of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1


Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
Gaseous ammonia chemically reacts with oxygen gas to produce nitrogen monoxide gas and water vapor....
Questions

Mathematics, 04.04.2021 18:10

English, 04.04.2021 18:10



English, 04.04.2021 18:10

Chemistry, 04.04.2021 18:10

Mathematics, 04.04.2021 18:10


Mathematics, 04.04.2021 18:10

Mathematics, 04.04.2021 18:10


Mathematics, 04.04.2021 18:10

Mathematics, 04.04.2021 18:10

Mathematics, 04.04.2021 18:10


Health, 04.04.2021 18:10

Business, 04.04.2021 18:10

Biology, 04.04.2021 18:10


Chemistry, 04.04.2021 18:10