
Chemistry, 20.07.2021 19:40 realneggalloyd
Gaseous BF3 and BCl3 are mixed in equal molar amounts. All B-F bonds have about the same bond enthalpy, as do all B-Cl bonds. Compare the numbers of microstates to explain why the mixture tends to react to form BF2Cl(g) and BCl2F(g

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

You know the right answer?
Gaseous BF3 and BCl3 are mixed in equal molar amounts. All B-F bonds have about the same bond enthal...
Questions



Spanish, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00


History, 16.12.2020 01:00




Biology, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00


Mathematics, 16.12.2020 01:00

Physics, 16.12.2020 01:00






English, 16.12.2020 01:00

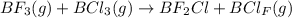
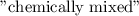 B halides has a
B halides has a 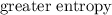 than a system of
than a system of 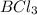 and
and 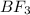 .
.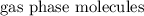 , but more distinguishable kinds of
, but more distinguishable kinds of 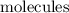 , hence, more microstates and higher entropy.
, hence, more microstates and higher entropy.

