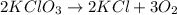Chemistry, 20.07.2021 01:00 mimithurmond03
When a mixture of potassium chlorate and an appropriate catalyst is heated in an open test tube, potassium chloride and oxygen gas are produced. This reaction is not reversible because a the reaction does not go to completion b the system reaches equilibrium c the system is closed d the oxygen escapes

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
When a mixture of potassium chlorate and an appropriate catalyst is heated in an open test tube, pot...
Questions

Biology, 16.12.2021 21:50

Law, 16.12.2021 21:50

Mathematics, 16.12.2021 21:50


Arts, 16.12.2021 21:50

Geography, 16.12.2021 21:50






English, 16.12.2021 21:50


Mathematics, 16.12.2021 21:50


Computers and Technology, 16.12.2021 21:50



Mathematics, 16.12.2021 21:50