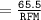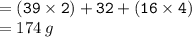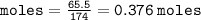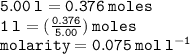Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3


You know the right answer?
Calculate the molarity of a solution consisting of 65.5 g of K2S0 4 in 5.00 L of solution. ...
Questions

Biology, 27.10.2020 04:50

Mathematics, 27.10.2020 04:50

Mathematics, 27.10.2020 04:50

Mathematics, 27.10.2020 04:50


Biology, 27.10.2020 04:50




Mathematics, 27.10.2020 04:50



Spanish, 27.10.2020 04:50

History, 27.10.2020 04:50

Mathematics, 27.10.2020 04:50

History, 27.10.2020 04:50

Physics, 27.10.2020 04:50


Mathematics, 27.10.2020 04:50