
Chemistry, 19.07.2021 01:00 whitakers87
using the balanced equation below how many grams of lead(||) sulfate would be produced from the complete reaction of 23.6 g lead (|V) oxide
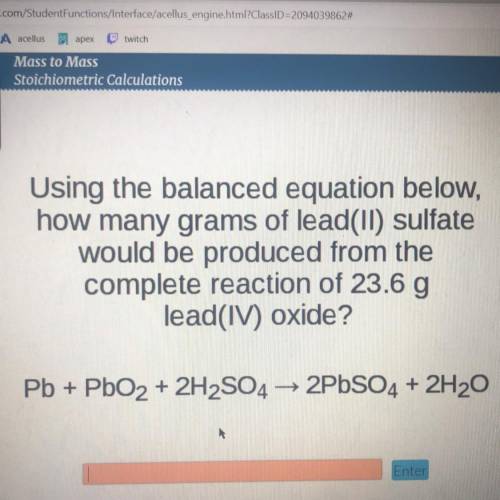

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
using the balanced equation below how many grams of lead(||) sulfate would be produced from the comp...
Questions

Physics, 18.02.2021 15:30

Mathematics, 18.02.2021 15:30


History, 18.02.2021 15:30

Advanced Placement (AP), 18.02.2021 15:30

Chemistry, 18.02.2021 15:30



Biology, 18.02.2021 15:30



Mathematics, 18.02.2021 15:30

Mathematics, 18.02.2021 15:30


History, 18.02.2021 15:30

Mathematics, 18.02.2021 15:30

Mathematics, 18.02.2021 15:30

Arts, 18.02.2021 15:30





