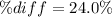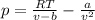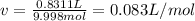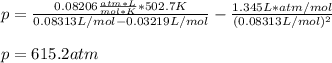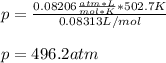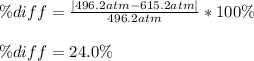Chemistry, 18.07.2021 03:50 andrea1704
According to the ideal gas law, a 9.998 mol sample of argon gas in a 0.8311 L container at 502.7 K should exert a pressure of 496.2
atm. What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For Ar
gas, a = 1.345 L’atm/mol? and b = 3.219x10-2 L/mol.
Pideal – Puan der Waals |
Percent difference
x 100

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1


You know the right answer?
According to the ideal gas law, a 9.998 mol sample of argon gas in a 0.8311 L container at 502.7 K s...
Questions


Mathematics, 10.09.2019 00:20

Mathematics, 10.09.2019 00:20


Mathematics, 10.09.2019 00:20





Mathematics, 10.09.2019 00:20


Physics, 10.09.2019 00:20

Mathematics, 10.09.2019 00:20

Mathematics, 10.09.2019 00:20

History, 10.09.2019 00:20

Mathematics, 10.09.2019 00:20

Mathematics, 10.09.2019 00:20