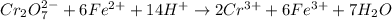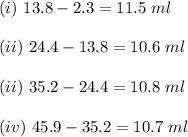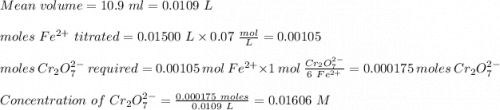Chemistry, 16.07.2021 20:00 jcazaresroman7308
ProblemWhat is the concentration of a tin(ll) chloride solution prepared from a sample of tin ore?Experimental DesignThe potassium dichromate solution is first standardized by titration with 15.00 mL of an acidified 0.07 mol/L solution of the primary standard, iron(II) ammonium sulfate-6-water. The standardized dichromate solution is then titrated against a sample of the acidified tin(II) chloride solution (You will do this step in the next question). Evidence TITRATION OF IRON(lI) SOLUTION(volume of K2Cr2O7(aq) required to react with 15.00 ml of 0.07 mol/L Fe2+(aq)) Trial1234Final buretreading(ml) 13.824.435.245.9Initial buretreading (ml) 2.313.824.435.2Find the concentration of the Cr2O72-(aq) in mol/L: (give your answer to 4 decimal places)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
ProblemWhat is the concentration of a tin(ll) chloride solution prepared from a sample of tin ore?Ex...
Questions


Computers and Technology, 03.03.2020 01:19

Mathematics, 03.03.2020 01:19



History, 03.03.2020 01:19



Mathematics, 03.03.2020 01:19



Mathematics, 03.03.2020 01:19

Mathematics, 03.03.2020 01:20

Mathematics, 03.03.2020 01:20

English, 03.03.2020 01:20

Mathematics, 03.03.2020 01:20

Mathematics, 03.03.2020 01:20



Mathematics, 03.03.2020 01:20