
Chemistry, 16.07.2021 01:50 needthehelp78
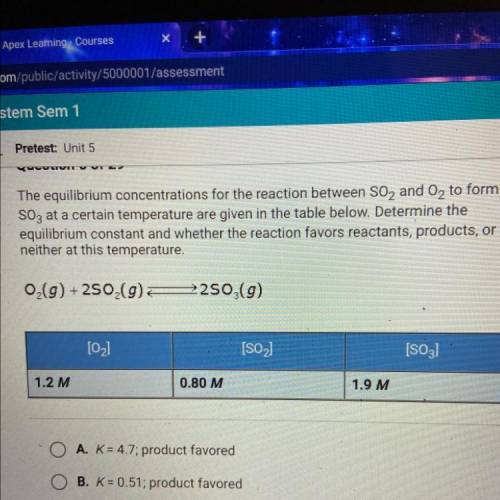
The equilibrium concentrations for the reaction between SO2 and O2 to form
SO3 at a certain temperature are given in the table below. Determine the
equilibrium constant and whether the reaction favors reactants, products, or
neither at this temperature.
O(g) +250 (9)
2250 (9)
[02]
[SO2)
[S03)
1.2 M
0.80 M
1.9 M
A. K = 4.7; product favored
B. K = 0.51; product favored
C. K = 0.51; reactant favored
o
D. K= 4.7; reactant favored

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
You know the right answer?
The equilibrium concentrations for the reaction between SO2 and O2 to form
SO3 at a certain tempera...
Questions

Computers and Technology, 23.07.2019 07:00

Mathematics, 23.07.2019 07:00


English, 23.07.2019 07:00


History, 23.07.2019 07:00

Mathematics, 23.07.2019 07:00

English, 23.07.2019 07:00


History, 23.07.2019 07:00

History, 23.07.2019 07:00

Business, 23.07.2019 07:00










