Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2
You know the right answer?
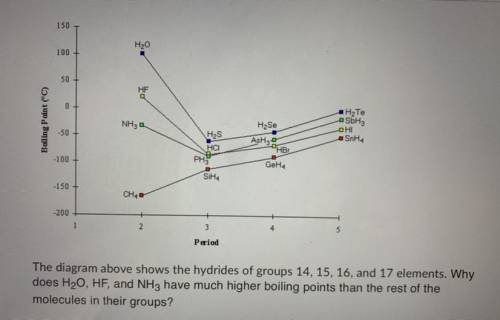
The diagram above shows the hydrides of groups 14, 15, 16, and 17 elements. Why does H20, HF, and NH...
Questions


Mathematics, 23.06.2020 10:57

English, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57


Health, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57


Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57



Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57



Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

History, 23.06.2020 10:57