
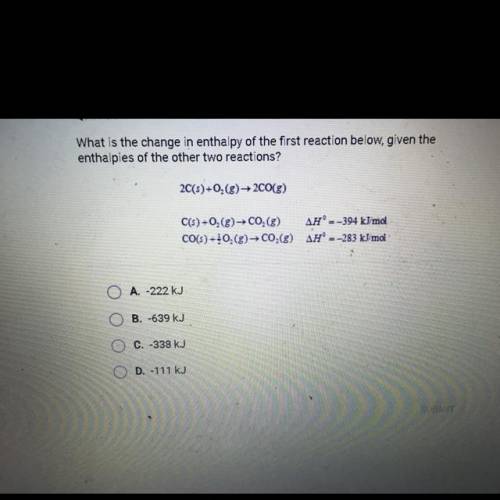
What is the change in enthalpy of the first reaction below, given the enthalpies of the other two reactions?
Here's the reactions:
There was a formatting issue with the specific chemistry symbols, there all correct in the picture below
2C(s) + O2(g) → 2CO(g)
C(s) + O2(g) → CO2(g) ∆H0= -394 KJ/mol
CO(s) + 1/2 O2(g) → CO2(g) ∆H0= -283 KJ/mol
There was a formatting issue with the specific chemistry symbols, there all correct in the picture below

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1


Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1
You know the right answer?
What is the change in enthalpy of the first reaction below, given the enthalpies of the other two re...
Questions

Business, 09.09.2021 14:00


Mathematics, 09.09.2021 14:00


Chemistry, 09.09.2021 14:00

Mathematics, 09.09.2021 14:00

Mathematics, 09.09.2021 14:00

Mathematics, 09.09.2021 14:00

Biology, 09.09.2021 14:00


English, 09.09.2021 14:00

Computers and Technology, 09.09.2021 14:00


History, 09.09.2021 14:00

History, 09.09.2021 14:00

Computers and Technology, 09.09.2021 14:00


History, 09.09.2021 14:00




