
In aqueous solution the Hg2+ ion forms a complex with four iodide anions. Write the formation constant expression for the equilibrium between the hydrated metal ion and the aqueous complex. Under that, write the balanced chemical equation for the first step in the formation of the complex.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
In aqueous solution the Hg2+ ion forms a complex with four iodide anions. Write the formation consta...
Questions


Mathematics, 09.08.2021 14:00


Mathematics, 09.08.2021 14:00

Physics, 09.08.2021 14:00






English, 09.08.2021 14:00


Health, 09.08.2021 14:00



English, 09.08.2021 14:00

World Languages, 09.08.2021 14:00

English, 09.08.2021 14:00


Mathematics, 09.08.2021 14:00

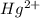 ion is used to represent a
ion is used to represent a ![[Hg(H_2O)_4]^{2+}](/tpl/images/1394/5126/65dfb.png) .
.
![[Hgl_4]^{2-}](/tpl/images/1394/5126/970b6.png) .
. ![[Hg(H_2O)_4]^{2+} +4I^{-} \to [HgI_4]^{2-}+4H_20](/tpl/images/1394/5126/c93e6.png)
![k_f=\frac{[HgI_4]^{2-}}{[Hg(H_2O)_4]^{2+} [I^{-}]^4}](/tpl/images/1394/5126/9ffc5.png)
![[Hg(H_2O)_4]^{2+} +I^{-} \to [HgI(H_2O)_3]^{+}+H_20](/tpl/images/1394/5126/c074d.png)


