Chemistry, 15.07.2021 14:50 triciajfive
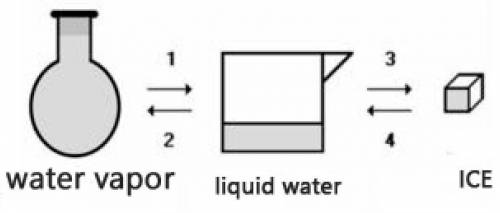
Consider the transformations a water sample undergoes without external pressure variation
(a) Transformations 2 and 4 are endothermic.
(b) Transformations 1 and 2 are exothermic.
(c) The amount of energy absorbed in 3 is equal to the amount released in 1.
(d) The amount of energy released in 2 is equal to the amount released in 4.
(e) The change of physical state does not involve heat energy.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

You know the right answer?
Consider the transformations a water sample undergoes without external pressure variation
(a) Trans...
Questions

Computers and Technology, 03.08.2019 23:30

Chemistry, 03.08.2019 23:30

History, 03.08.2019 23:30

Computers and Technology, 03.08.2019 23:30

English, 03.08.2019 23:30

Chemistry, 03.08.2019 23:30

Computers and Technology, 03.08.2019 23:30



Mathematics, 03.08.2019 23:30

Mathematics, 03.08.2019 23:30

Mathematics, 03.08.2019 23:30








English, 03.08.2019 23:30