
Chemistry, 15.07.2021 07:10 xonyemaa12
The equilibrium concentrations for the reaction between SO2 and O2 to form SO3 at a certain temperature are given in the table below. Determine the equilibrium constant and whether the reaction favors reactants, products, or neither at this temperature.
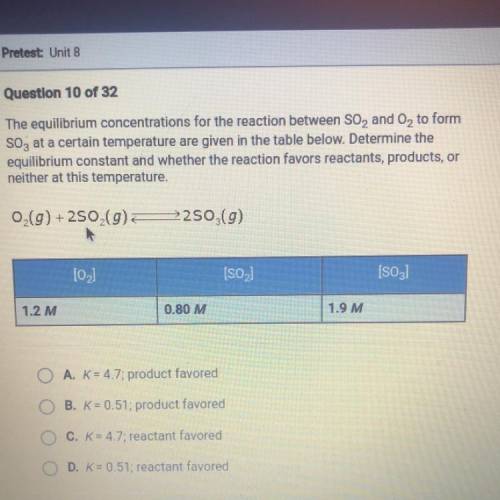

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
The equilibrium concentrations for the reaction between SO2 and O2 to form SO3 at a certain temperat...
Questions

English, 20.09.2020 04:01





English, 20.09.2020 04:01

History, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01



Mathematics, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01



SAT, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01




