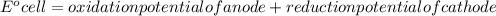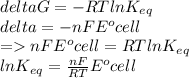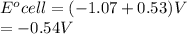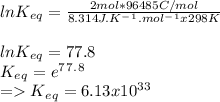Chemistry, 14.07.2021 20:10 SkyeShadow525
Determine the equilibrium constant, Keq, at 25°C for the reaction
2Br- (aq) + I2(s) <--> Br2(l) + 2I- (aq)
Eocell = (0.0257/n) lnKeq, Calculate Eocell from Use this equation to calculate K value.
Eo (I2/I-) = +0.53, Eo (Br2/Br-) = +1.07,

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Determine the equilibrium constant, Keq, at 25°C for the reaction
2Br- (aq) + I2(s) <--> Br2(...
Questions




Mathematics, 08.09.2020 07:01


Mathematics, 08.09.2020 07:01



Mathematics, 08.09.2020 07:01




English, 08.09.2020 07:01

Physics, 08.09.2020 07:01

Mathematics, 08.09.2020 07:01

Mathematics, 08.09.2020 07:01

Mathematics, 08.09.2020 07:01

World Languages, 08.09.2020 07:01

Mathematics, 08.09.2020 07:01

English, 08.09.2020 07:01