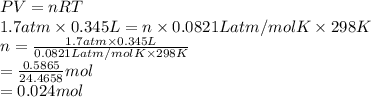Chemistry, 14.07.2021 01:00 hcarcel8287
An ideal gas has a temperature of 25 oC, a pressure of 1.7 atm, and volume of 345 mL, how many moles of gas are present in the container?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1


You know the right answer?
An ideal gas has a temperature of 25 oC, a pressure of 1.7 atm, and volume of 345 mL, how many moles...
Questions


English, 30.10.2020 21:40





Mathematics, 30.10.2020 21:40





Chemistry, 30.10.2020 21:40

Mathematics, 30.10.2020 21:40



Mathematics, 30.10.2020 21:40


Mathematics, 30.10.2020 21:40


Mathematics, 30.10.2020 21:40

 = (25 + 273) K = 298 K
= (25 + 273) K = 298 K