

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

You know the right answer?
Compound X has a molar mass of 266.64 g/mol and the following composition: aluminum 20.24% chlorine...
Questions

Mathematics, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40







History, 01.12.2020 18:40



Mathematics, 01.12.2020 18:40

Spanish, 01.12.2020 18:40





Mathematics, 01.12.2020 18:40


English, 01.12.2020 18:40

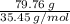 = 2.25 mol of chlorine
= 2.25 mol of chlorine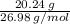 = 0.750 mol of Al.
= 0.750 mol of Al.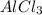 .
.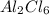 .
.

