Chemistry, 10.07.2021 04:10 preguntassimples
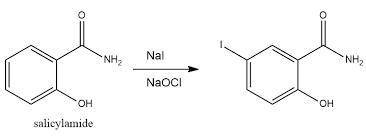
To conduct the synthesis of iodosalicylamide, Edward used 1.07 g of salicylamide (MW: 137.14 g/mol) and 1.68 g of sodium iodide (MW:149.89 g/mol). Assuming the reaction yield is 100%, how many grams of iodosalicylamide (MW:263.03 g/mol) would be formed

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1


Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

You know the right answer?
To conduct the synthesis of iodosalicylamide, Edward used 1.07 g of salicylamide (MW: 137.14 g/mol)...
Questions



Mathematics, 04.05.2021 21:20



Mathematics, 04.05.2021 21:20

Arts, 04.05.2021 21:20



Mathematics, 04.05.2021 21:20

Mathematics, 04.05.2021 21:20

Mathematics, 04.05.2021 21:20




Mathematics, 04.05.2021 21:20


Mathematics, 04.05.2021 21:20

Mathematics, 04.05.2021 21:20