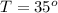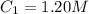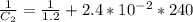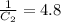Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
The rate constant for a particular second order reaction is 2.4 x 10-2 M-1 s-1 at 35∘C. If the initi...
Questions


Mathematics, 10.04.2020 20:54

Social Studies, 10.04.2020 20:54

Mathematics, 10.04.2020 20:54




Mathematics, 10.04.2020 20:54


Computers and Technology, 10.04.2020 20:54



Computers and Technology, 10.04.2020 20:54

History, 10.04.2020 20:54


Mathematics, 10.04.2020 20:54



Mathematics, 10.04.2020 20:55