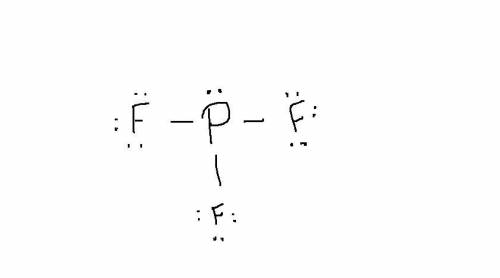Chemistry, 10.07.2021 01:20 jellyangie1
Write a Lewis structure for the phosphorus trifluoride molecule, PF3. Draw the Lewis dot structure for PF3. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1

Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
You know the right answer?
Write a Lewis structure for the phosphorus trifluoride molecule, PF3. Draw the Lewis dot structure f...
Questions


Advanced Placement (AP), 06.01.2021 21:20


Mathematics, 06.01.2021 21:20







Mathematics, 06.01.2021 21:20



Health, 06.01.2021 21:20


History, 06.01.2021 21:20

Mathematics, 06.01.2021 21:20