
Chemistry, 09.07.2021 21:00 faithrawlins14
If 36.0 mL of 0.20 M HCl is added to 30.0 mL of 0.40 M NaOH, what will be the pH of the resulting solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
If 36.0 mL of 0.20 M HCl is added to 30.0 mL of 0.40 M NaOH, what will be the pH of the resulting so...
Questions

Mathematics, 05.11.2020 22:10


Mathematics, 05.11.2020 22:10

Physics, 05.11.2020 22:10

Social Studies, 05.11.2020 22:10

Chemistry, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10


Arts, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

English, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10



History, 05.11.2020 22:10

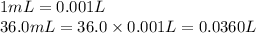
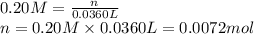

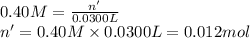
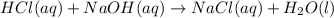
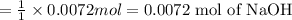
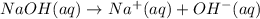
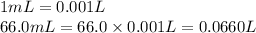
![[OH^-]=\frac{0.0048 mol}{0.0660 L}=0.073 M](/tpl/images/1391/7967/4b203.png)
![pOH=-\log[OH^-]\\pOH=-\log[0.073 M]=1.1\\14 = pH + pOH\\pH = 14 - pOH = 14 - 1.1 = 12.9](/tpl/images/1391/7967/b9345.png)

![[H^+]=[HCl]](/tpl/images/1391/7967/f6a88.png)
![[OH^-]=[NaOH]](/tpl/images/1391/7967/90eec.png)


