
Chemistry, 09.07.2021 18:10 nalanisancruzado
A solution contains one or more of the following ions: Ag , Ca2 , and Co2 . Lithium bromide is added to the solution and no precipitate forms. An excess of lithium sulfate is then added to the solution and a precipitate forms. The precipitate is filtered off and lithium phosphate is added to the remaining solution, producing a precipitate. Which ions are present in the original solution

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1


You know the right answer?
A solution contains one or more of the following ions: Ag , Ca2 , and Co2 . Lithium bromide is added...
Questions



Mathematics, 29.04.2021 05:00

Mathematics, 29.04.2021 05:00

Mathematics, 29.04.2021 05:00

Mathematics, 29.04.2021 05:00


Biology, 29.04.2021 05:00

Mathematics, 29.04.2021 05:00



English, 29.04.2021 05:00



Mathematics, 29.04.2021 05:00

Mathematics, 29.04.2021 05:10

Biology, 29.04.2021 05:10



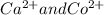
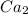 and
and 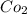
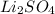 to it precipitation takes place.
to it precipitation takes place.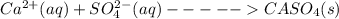
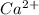 is present
is present 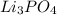 is added again precipitation takes place.
is added again precipitation takes place.


