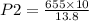Chemistry, 09.07.2021 16:30 annrawcliffe
A 10.0 L balloon contains helium gas at a pressure of 655 mmHg. What is the final pressure, in millimeters of mercury, if the final volume is 13.8 L? Please show your work in order to receive credit.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3



Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
A 10.0 L balloon contains helium gas at a pressure of 655 mmHg. What is the final pressure, in milli...
Questions

Mathematics, 03.06.2021 15:20


Mathematics, 03.06.2021 15:20

Mathematics, 03.06.2021 15:20





Mathematics, 03.06.2021 15:20


Biology, 03.06.2021 15:20

Biology, 03.06.2021 15:20

SAT, 03.06.2021 15:20


Mathematics, 03.06.2021 15:20

Mathematics, 03.06.2021 15:20

Business, 03.06.2021 15:20

Computers and Technology, 03.06.2021 15:20

Computers and Technology, 03.06.2021 15:20

Biology, 03.06.2021 15:20