
Chemistry, 09.07.2021 03:20 yakshp3702
During a reaction, ΔH for reactants is −750 kJ/mol and ΔH for products is 920 kJ/mol. Which statement is correct about the reaction? (5 points)
Group of answer choices
It is endothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
It is endothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.
It is exothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
It is exothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

You know the right answer?
During a reaction, ΔH for reactants is −750 kJ/mol and ΔH for products is 920 kJ/mol. Which statemen...
Questions


Computers and Technology, 21.12.2020 17:10



English, 21.12.2020 17:10


Law, 21.12.2020 17:10

Mathematics, 21.12.2020 17:10


Mathematics, 21.12.2020 17:10



Mathematics, 21.12.2020 17:10



Mathematics, 21.12.2020 17:10

Social Studies, 21.12.2020 17:10

English, 21.12.2020 17:10


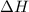 is always negative.
is always negative.

