Chemistry, 08.07.2021 21:50 jjiopppotdd5638
For a reaction, AH = 176 kJ/mol and A SO = 0.285 kJ/(K•mol). At what
temperatures is this reaction spontaneous?
A. At no temperature
B. T< 50 K
C. T>617 K
D. T< 617 K

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1


Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
For a reaction, AH = 176 kJ/mol and A SO = 0.285 kJ/(K•mol). At what
temperatures is this reaction...
Questions

Mathematics, 05.06.2020 18:59


Mathematics, 05.06.2020 18:59

Social Studies, 05.06.2020 18:59

Biology, 05.06.2020 18:59

English, 05.06.2020 18:59




Mathematics, 05.06.2020 18:59



Mathematics, 05.06.2020 18:59

Mathematics, 05.06.2020 18:59


Mathematics, 05.06.2020 18:59



History, 05.06.2020 18:59

Mathematics, 05.06.2020 18:59

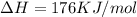
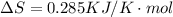
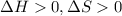
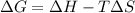
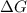 is negative, then the reaction is spontaneous.
is negative, then the reaction is spontaneous.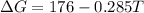
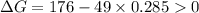
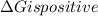 .Hence, the reaction is not spontaneous.
.Hence, the reaction is not spontaneous.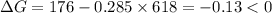
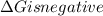 .Hence, the reaction is spontaneous.
.Hence, the reaction is spontaneous.