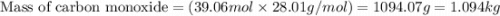Chemistry, 08.07.2021 18:50 leilaford2003
What mass of carbon monoxide is needed to react with 2.08 kg of iron oxide? > Fe^2O^3 + 3CO --> 2Fe + 3CO^2
20 points, plz help and plz show how you work
I WILL MARK YOU BRAINIEST IF U ANSWER WITH FULL WORKING OUT!

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
What mass of carbon monoxide is needed to react with 2.08 kg of iron oxide? > Fe^2O^3 + 3CO -->...
Questions

Health, 30.09.2020 04:01

Physics, 30.09.2020 04:01

Mathematics, 30.09.2020 04:01




Mathematics, 30.09.2020 04:01



Chemistry, 30.09.2020 04:01




Mathematics, 30.09.2020 04:01


Biology, 30.09.2020 04:01

Mathematics, 30.09.2020 04:01



Biology, 30.09.2020 04:01

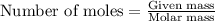 ......(1)
......(1)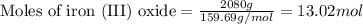
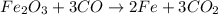
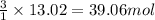 of CO
of CO