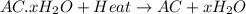A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g after heating. If the molar mass of the anhydrous compound (AC) is
195.5 g/mol, what is the water of crystallization for the formula of the unknown
hydrate?
Type your work for partial credit.
Answer choices: 2, 3, 5, or 6.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2


Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g af...
Questions

Geography, 23.07.2019 04:50

Health, 23.07.2019 04:50

Computers and Technology, 23.07.2019 04:50


Social Studies, 23.07.2019 04:50



Business, 23.07.2019 04:50

History, 23.07.2019 04:50

Mathematics, 23.07.2019 04:50









Business, 23.07.2019 04:50

Social Studies, 23.07.2019 04:50