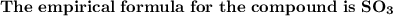Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1
You know the right answer?
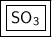
Calculate the Empirical Formula for the following compound:
0.300 mol of S and 0.900 mole of O...
0.300 mol of S and 0.900 mole of O...
Questions





Mathematics, 05.01.2020 20:31


Mathematics, 05.01.2020 20:31


Mathematics, 05.01.2020 20:31

Business, 05.01.2020 20:31

Biology, 05.01.2020 20:31




Chemistry, 05.01.2020 20:31

Mathematics, 05.01.2020 20:31

Mathematics, 05.01.2020 20:31

History, 05.01.2020 20:31