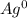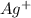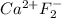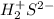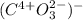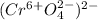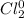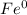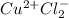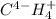Assign oxidation state to each atom in each element ion or compound.
a. Ag
b. Ag+
c. Ca...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
Questions


Chemistry, 27.10.2020 20:30


Mathematics, 27.10.2020 20:30



Mathematics, 27.10.2020 20:30

Mathematics, 27.10.2020 20:30

Computers and Technology, 27.10.2020 20:30





Mathematics, 27.10.2020 20:30



Mathematics, 27.10.2020 20:30


Geography, 27.10.2020 20:30