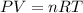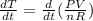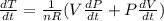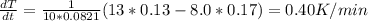Chemistry, 06.07.2021 22:40 ralphmillerrr
The gas law for an ideal gas at absolute temperature T (in kelvins), pressure P (in atmospheres), and volume V (in liters) is PV = nRT, where n is the number of moles of the gas and R = 0.0821 is the gas constant. Suppose that, at a certain instant, P = 8.0 atm and is increasing at a rate of 0.13 atm/min and V = 13 L and is decreasing at a rate of 0.17 L/min. Find the rate of change of T with respect to time (in K/min) at that instant if n = 10 mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1

Chemistry, 23.06.2019 06:30
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1

Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
You know the right answer?
The gas law for an ideal gas at absolute temperature T (in kelvins), pressure P (in atmospheres), an...
Questions






Mathematics, 24.06.2019 09:30


World Languages, 24.06.2019 09:30

Chemistry, 24.06.2019 09:30




English, 24.06.2019 09:30

Mathematics, 24.06.2019 09:30



History, 24.06.2019 09:30

Mathematics, 24.06.2019 09:30

Chemistry, 24.06.2019 09:30