Using the balanced equation below,
how many grams of sodium
thiosulfate would be required to<...

Chemistry, 06.07.2021 19:00 destinyranson
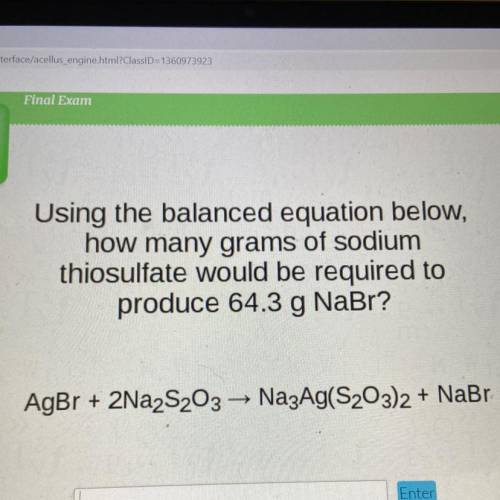
Using the balanced equation below,
how many grams of sodium
thiosulfate would be required to
produce 64.3 g NaBr?
AgBr + 2Na2S203 — Na3Ag(S203)2 + NaBr

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Imagine that you own a property that is exactly 2.2 acres large. you want to sell your property, but your realtor tells you that you cannot sell your land by the acre. in order to sell your land you need to determine the area you own in units of square meters? given that there are 1.6 kilometers in 1 mile and 640 acres in 1 square mile, what is the area of land that you own in square meters square meters?
Answers: 2

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Questions

Chemistry, 18.09.2019 03:50


Geography, 18.09.2019 03:50

English, 18.09.2019 03:50

History, 18.09.2019 03:50



Computers and Technology, 18.09.2019 03:50

Mathematics, 18.09.2019 03:50

Mathematics, 18.09.2019 03:50



Social Studies, 18.09.2019 03:50







Arts, 18.09.2019 03:50



